|
|
|
|
Title: Air/Fuel Ratio
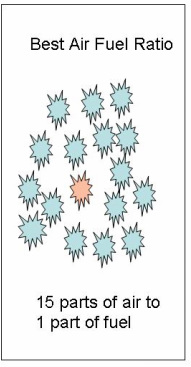
Description of Principle: Air-fuel ratio is an important principle in the study of gasoline engines. Air-fuel ratio is defined as the ratio of the amount of air to fuel that is mixed by the carburetor or fuel injectors. The most efficient air-fuel ratio is called the stoichiometric ratio. The air and fuel must be thoroughly mixed in combustion. Each molecule of fuel must have enough air surrounding it to completely burn the mixture. If the two are not mixed in the correct ratio, the engine efficiency will drop and exhaust emission levels will increase. The standard air-fuel ratio should be near 15 parts of air to 1 part of fuel. The most efficient ratio is stated as 14.7 to 1. For every pound of fuel used, 14.7 pounds of air is needed. See the graphic representation above. In terms of volume, this is equal to burning 1 gallon of fuel in 9,000 gallons of air. Although 14.7 to 1 is the most efficient air-fuel ratio, there are times when this ratio is not effective. For example, more fuel is needed during cold starting and during acceleration of the vehicle.
Description of Principle: Air-fuel ratio is an important principle in the study of gasoline engines. Air-fuel ratio is defined as the ratio of the amount of air to fuel that is mixed by the carburetor or fuel injectors. The most efficient air-fuel ratio is called the stoichiometric ratio. The air and fuel must be thoroughly mixed in combustion. Each molecule of fuel must have enough air surrounding it to completely burn the mixture. If the two are not mixed in the correct ratio, the engine efficiency will drop and exhaust emission levels will increase. The standard air-fuel ratio should be near 15 parts of air to 1 part of fuel. The most efficient ratio is stated as 14.7 to 1. For every pound of fuel used, 14.7 pounds of air is needed. See the graphic representation above. In terms of volume, this is equal to burning 1 gallon of fuel in 9,000 gallons of air. Although 14.7 to 1 is the most efficient air-fuel ratio, there are times when this ratio is not effective. For example, more fuel is needed during cold starting and during acceleration of the vehicle.
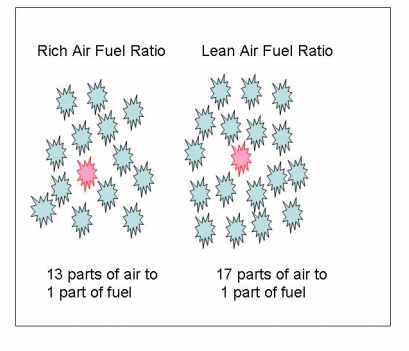
As graphically shown on the left, a low ratio of around 13 to 1 indicates a rich mixture of fuel. A mixture of 17 to 1 indicates a lean mixture. Generally, rich mixtures are less efficient during combustion. A rich mixture is used during cold weather and starting conditions. A lean mixture burns hotter than a rich mixture. This is because as fuel enters the combustion chamber, it acts as a coolant to the combustion. With less fuel to cool the combustion, the combustion temperatures get hotter. This condition can cause severe damage to the pistons and valves if not corrected.
Back to Mechanical Principles
Back to Mechanical Principles
|
|
|
|