Title: Atomic Structure
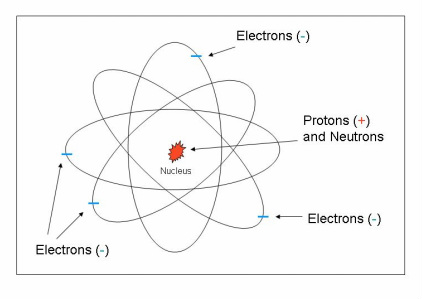
Description: The heart of all information about electricity is in the study of atoms and atomic structure. Everything, such as water, trees, buildings, etc. is made up of atoms. They are very small, about a millionth of an inch across. There are millions of atoms in a single breath of air. The structure of an atom is illustrated in the figure to the left. Each atom has at its center a nucleus that contains both protons and neutrons. The nucleus is the major part of the atom. Protons are said to carry a positive charge (+). Neutrons carry no charge and are not considered in the study of electricity. Also present in the atom are electrons, which orbit around the nucleus. Electrons are very light in comparison to the nucleus. They carry a negative
charge (-).
Description: The heart of all information about electricity is in the study of atoms and atomic structure. Everything, such as water, trees, buildings, etc. is made up of atoms. They are very small, about a millionth of an inch across. There are millions of atoms in a single breath of air. The structure of an atom is illustrated in the figure to the left. Each atom has at its center a nucleus that contains both protons and neutrons. The nucleus is the major part of the atom. Protons are said to carry a positive charge (+). Neutrons carry no charge and are not considered in the study of electricity. Also present in the atom are electrons, which orbit around the nucleus. Electrons are very light in comparison to the nucleus. They carry a negative
charge (-).
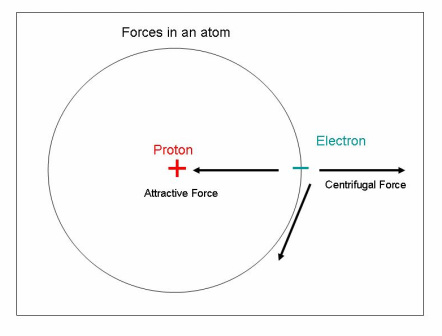
There is an attraction between the negative electrons and the positive protons. The attractive force and the centrifugal forces cause the electrons to orbit the nucleus or protons in the center. This is illustrated in the drawing to the left.
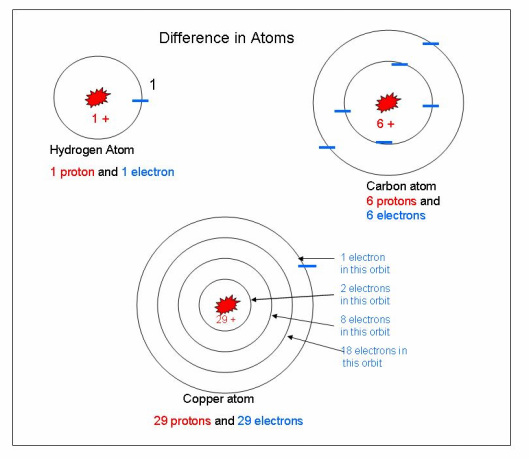
The number of electrons in all orbits and the number of protons in the nucleus will try to remain equal. If they are equal, the atom is said to be balanced or neutral. The illustration to the left shows several atoms with the number of protons and electrons shown for comparison. The Hydrogen atom has only 1 proton and 1 electron in the atom. Carbon has 6 protons and 6 electrons in the atom. Copper has 29 protons and 29 electrons in the atom.
When studying electricity the electrons in the outer orbit are the only ones we need to study. The outer orbit of the atom is called the valence ring. It holds the outer most electrons. Electrons in the valence ring can easily be added or removed. The copper atom to the left has only 1 electron in its valence ring. Also, an atom with several electrons missing will try to gain or capture other electrons in order to balance itself. If an atom has an excess amount of electrons in the valence ring, it is said to be negatively charged and will try to get rid of these electrons. This will also help balance the atom.
Certain materials can lose or gain electrons rather easily from the valence ring. This depends on the number of electrons needed in the valence ring to balance the atom. If an atom loses electrons easily, the material is called a good conductor. If the atom cannot lose electrons easily, the material is called a good insulator. So insulators and conductors are defined as follows:
--A material with three or fewer electrons in the outer orbit is called a good conductor.
--A material with five or more electrons in the outer orbit is called a good insulator.
--A material with only four electrons in the outer orbit is called a semiconductor.
Materials that have only four electrons in the outer orbit can be either a conductor or an insulator, depending upon what is done to it in the circuit. These materials are called semiconductors. They are used in computer circuits with components such as transistors, diodes, Zener diodes, etc.
Relationship to Street Rods: There are many electrical circuits in a typical street rod. These circuits use mostly copper wire (or other good conductor materials) to move the electrons around the circuit. Copper wire is a good conductor. It only has one electron in the outer orbit so it can be moved easily. In an electrical circuit, the electricity that is flowing is actually the electrons moving from one atom to another. These electrons move at the speed of light or 186,000 miles per second. When you see a spark plug fire or a spark between the battery and a wire, you are actually seeing millions and millions of electrons jumping across the open circuit.
Back to Electrical Principles
When studying electricity the electrons in the outer orbit are the only ones we need to study. The outer orbit of the atom is called the valence ring. It holds the outer most electrons. Electrons in the valence ring can easily be added or removed. The copper atom to the left has only 1 electron in its valence ring. Also, an atom with several electrons missing will try to gain or capture other electrons in order to balance itself. If an atom has an excess amount of electrons in the valence ring, it is said to be negatively charged and will try to get rid of these electrons. This will also help balance the atom.
Certain materials can lose or gain electrons rather easily from the valence ring. This depends on the number of electrons needed in the valence ring to balance the atom. If an atom loses electrons easily, the material is called a good conductor. If the atom cannot lose electrons easily, the material is called a good insulator. So insulators and conductors are defined as follows:
--A material with three or fewer electrons in the outer orbit is called a good conductor.
--A material with five or more electrons in the outer orbit is called a good insulator.
--A material with only four electrons in the outer orbit is called a semiconductor.
Materials that have only four electrons in the outer orbit can be either a conductor or an insulator, depending upon what is done to it in the circuit. These materials are called semiconductors. They are used in computer circuits with components such as transistors, diodes, Zener diodes, etc.
Relationship to Street Rods: There are many electrical circuits in a typical street rod. These circuits use mostly copper wire (or other good conductor materials) to move the electrons around the circuit. Copper wire is a good conductor. It only has one electron in the outer orbit so it can be moved easily. In an electrical circuit, the electricity that is flowing is actually the electrons moving from one atom to another. These electrons move at the speed of light or 186,000 miles per second. When you see a spark plug fire or a spark between the battery and a wire, you are actually seeing millions and millions of electrons jumping across the open circuit.
Back to Electrical Principles